Fenestrated Endovascular Aortic Aneurysm Repair (FEVAR)
Louisville’s Surgical Care Associates Vascular Surgery Team is among the first in the nation to offer fenestrated endografts for treating complex abdominal aortic aneurysms, and the only hospital in the area currently doing so. Recently FDA-approved, the Zenith Fenestrated AAA Endovascular Graft by Cook Medical offers a minimally invasive treatment option for patients whose aneurysm anatomy is incompatible with existing devices. This will enable treatment of 15 to 20 percent more AAA patients who previously were ineligible for traditional endovascular repair. “This represents the next step forward in minimally invasive aneurysm therapy and the biggest advance within the last five years,” says Dr. Thomas Bergamini.
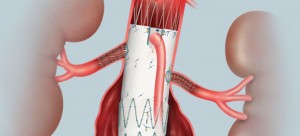
The fenestrated endograft is the world’s first to incorporate tailored openings, or fenestrations, in the
endograft’s top section. This enables the self-expanding, fabric-covered stent-graft to treat aortic and
aortoiliac aneurysms extending close to the renal and superior mesenteric arteries, which are then stented to reduce the risk of restricting or blocking blood flow to the kidneys and bowel. Each fenestrated device is custom-made from a spiral CT-generated 3-D computer model of the patient’s anatomy. The fenestrations in the stent-graft are precisely positioned where the patient’s renal
or bowel arteries branch off.
“We are very enthusiastic about bringing this new technology to Louisville and the significant number of patients who previously were not candidates for minimally invasive aneurysm repair,” says Bradley G. Thomas, MD, of Surgical Care Associates. “They can now enjoy the benefits of decreased complications, early discharge and a quick return to a normal quality of life.”